Home › Forum › Ask A Member › Transom wood › Re: Transom wood
Gentlemen (and Ladies):
I am new to this forum, but I am not at all new to materials. I have followed discussions of transoms on this and tinboats, and I think that all of you might want to be reminded about what you learned in High School chemistry – the electromotive series of metals.
Yes, Pressure Treated (PT) plywood will cause accelerated corrosion of aluminum. PT pine plywood is infused with copper compounds; copper is anti-fungal, which is what you want in a 4X4 post in the ground. The most common pressure-treating in bygone years was CCA – Chromated Copper Arsenate. The copper compounds were to resist rot, the arsenic kept bugs from eating your fenceposts. It worked great. But the American Wood Protection Association (AWPA), in their wisdom, decided, with encouragement by the EPA, to eliminate the arsenic. Pressure-treating industry went to MCA, Micronized Copper Azole. But it still contains copper, lots of it; you have to use stainless or ceramic-coated fasteners with it, galvanized won’t survive.
If you want to read more about recent pressure treating, go here: https://en.wikipedia.org/wiki/Wood_pres … technology
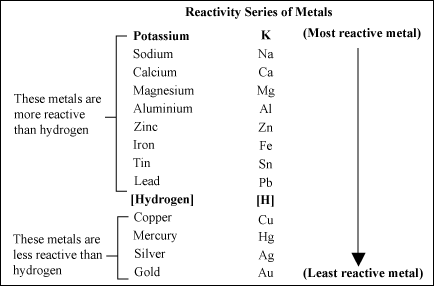
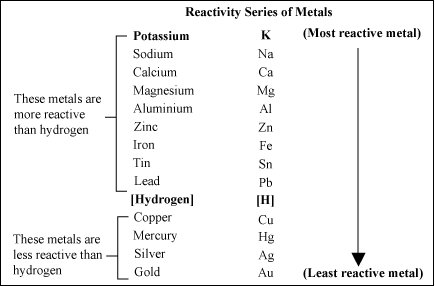
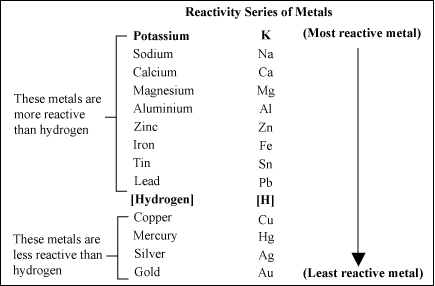
Here is the Activity Series for common metals;
https://d2gne97vdumgn3.cloudfront.net/a … 29EBuActGX
To understand it, the metals lower on the series are more “noble” than those above it, meaning that when two are in contact with each other, they set up an electrochemical cell, and the lesser of the two is corroded. This is the reason for zinc-plating steel; the zinc becomes the sacrificial anode, and corrodes before the steel can. The Al-Can pipeline has plates of zinc attached to it to slow corrosion of the steel pipe. So when aluminum is in contact with copper (or any copper alloy, like brass or bronze), it is the aluminum that will corrode. The action is accelerated in the presence of water, even more so in salt water. See the hydrogen between copper and lead? Water is an electrolyte. Going from top to bottom, the metals lose electrons more readily to form positive ions – this is the way batteries work. Dissimilar metals in an electrolyte = electricity. The less noble metal is consumed to give up its electrons.
And George? The fault is not the pine in plywood. Because it is cross-laminated, plywood, any plywood is stronger, pound for pound, than almost any solid hardwood. (That doesn’t mean that it is harder – strength and hardness are two separate things) The problem is that pine, Baltic birch, any plywood you can name, will not resist rot unless it is pressure-treated.
I am always amazed that people on forums don’t really know anything about the chemistry of the materials they are thinking of using on their boats, and thus cannot make informed judgement of the product to use. They go by anecdotal recommendation, maybe twice or thrice removed. And that goes for finishes, as well.
No, I am not a chemist; I just know something about the practical chemistry of objects.